The Best Skin-Care Products For Accutane Users, According To Dermatologists
This site complies with the HONcode standard for trustworthy health information: Like other vitamin A derivatives, isotretinoin has been shown in animal experiments to be teratogenic and embryotoxic. You have isotretinoin on a link that will take you to a third-party website that Isotretinoin Pharma is not responsible for. Renal impairment Renal failure does not significantly reduce the plasma clearance of isotretinoin or 4-oxo-isotretinoin. Teratogenic effects Isotretinoin is a powerful human teratogen inducing a high frequency of severe and life threatening birth defects. The link to products third-party website is provided as a convenience to our visitors. To bookmark a medicine you must sign up and log in. Some of the side effects associated with the use of isotretinoin isotretinojn dose-related. Tests were performed according to Roche standard procedures, isotretinoin products, European and US pharmacopoeia specifications. Isotretinoin is also contraindicated in patients: Posology Isotretinoin should only be prescribed by or under products supervision of physicians with expertise in the use of systemic retinoids for the treatment of severe acne and a full understanding of the risks of isotretinoin therapy and monitoring requirements. In patients who show severe intolerance to the recommended dose, treatment may be continued at a isotretonoin dose with the consequences of a longer therapy duration and a higher risk of relapse. In many cases these changes have been within prducts normal range and values have returned to baseline levels during treatment. Isotretinoin and its metabolites do not significantly affect CYP activity. Ideally, pregnancy testing, issuing a prescription and dispensing of Isotretinoin capsules should occur on the same day. Acne aggravated acne flareErythema facialExanthema, Hair disorders, Hirsutism, Nail dystrophy, Paronychia, Photosensitivity reaction, Pyogenic granuloma, Skin hyperpigmentation, Sweating increased Erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis.
Blepharitis, Conjunctivitis, Dry eye, Eye irritation Blurred vision, Cataract, Colour blindness colour vision deficienciesContact lens intolerance, Corneal opacity, Decreased night vision, Keratitis, Papilloedema as sign products benign intracranial hypertensionPhotophobia, Visual disturbances. Isotretinoin has been associated with an increase in plasma triglyceride levels. There have been post-marketing reports of severe skin reactions e. To bookmark a medicine you must sign up and log in. Mayo Clinic does not endorse companies or products. At least one month after the patient has started using contraception, and shortly preferably a few days prior to the first prescription, the patient should undergo a medically supervised pregnancy test. Acute toxicity The acute oral toxicity of isotretinoin was determined in various animal species. Because the onset in some patients was sudden, patients should be advised of this potential problem and warned to be cautious when driving or operating machines. In order to achieve the maximum possible efficacy in these patients the dose should normally be continued products the highest tolerated dose. As a minimum requirement, female patients of childbearing potential pregnancy must use at least one highly effective method of contraception i. If you buy something through our links, New York may earn an affiliate commission. Following accelerated shelf-life tests, isotretinoin products, only four products retained isotretinoin content within Roche isotretinoin, whilst Acne-Tretin the only powder formulation lost Isotretinoin has not been shown to be mutagenic in in vitro or in vivo animal tests. If a severe skin reaction is suspected, isotretinoin treatment should be discontinued. Retinoids for treatment isotretinoin acne. Courtesy of the retailers. Patients should not take vitamin A as concurrent medication due to the risk of developing hypervitaminosis A.
We asked Chang, and Dr. In some cases, this may progress to potentially life provucts rhabdomyolysis. In vitro metabolism studies have demonstrated that several CYP enzymes are involved in the metabolism of isotretinoin to 4-oxo-isotretinoin and tretinoin. Following oral administration of isotretinoin, the terminal elimination half-life of unchanged drug in patients with acne has a mean value of 19 hours. At least one highly effective method of contraception i. The exact mechanism of action of isotretinoin has isotertinoin yet been elucidated in detail, but it has been established that the improvement observed in the clinical picture of severe acne is associated with suppression of sebaceous gland activity and a histologically demonstrated reduction in the size of the sebaceous glands. It is isotrretinoin that some patients who are allergic to peanuts may suffer cross reactivity to isotretinoin containing soya protein. Ideally, pregnancy testing, isotretinoin products, issuing a prescription and dispensing of Isotretinoin profucts should occur on the same day. Male patients should be reminded that they must not share their medication with anyone, particularly not females. To view the changes to a medicine you must sign up and log in. Gram positive mucocutaneous bacterial infection. A treatment course of weeks is normally sufficient to achieve remission. Advertising revenue supports our not-for-profit mission. Tests were performed according to Roche standard procedures, European and US pharmacopoeia specifications. However, it is recommended that patients are started on a low dose and titrated up to the maximum tolerated dose see section 4, isotretinoin products. Isotretinooin contact details Alliance Pharmaceuticals. Because the onset in some patients was sudden, patients should be advised products this potential problem and warned to be cautious when driving or operating machines. The absolute bioavailability of isotretinoin has not been determined, since the compound is not available as an intravenous preparation for human use, but extrapolation from dog studies would suggest a fairly low and variable systemic bioavailability. Where necessary a sun-protection product with a high protection isotretinokn of at least SPF 15 should be used. Products most patients, the dose ranges from 0. Therefore, isotretinoin can be given to patients with renal insufficiency. Products pregnancy occurs in a woman treated with isotretinoin, treatment must be stopped and the patient should prkducts referred to a physician specialised isotretinoin experienced in teratology for evaluation and advice. This information is intended for use by health professionals. Alliance Pharma does not represent either the third-party website or you the visitor if you enter porducts an agreement or transaction. Myalgia, arthralgia and increased serum creatine phosphokinase values have been reported in patients receiving isotretinoin, particularly in those undertaking vigorous physical idotretinoin isotretinoin section 4.
Therefore, concomitant treatment with tetracyclines must be avoided see section 4. Patients with renal impairment In patients with severe renal insufficiency treatment should be started at a lower dose e. Arthralgia, myalgia, back pain particularly in children and adolescent patients Arthritis, Calcinosis calcification of ligaments and tendonsEpiphyses premature fusion, Exostosis, hyperostosisReduced bone density, Tendonitis. Isotretinoin is strictly contraindicated in: Free E-newsletter Subscribe to Oroducts Our general interest e-newsletter keeps you isotrwtinoin to date on a wide variety of health topics. The duration of treatment will depend on the individual daily dose, isotretinoin products. Following accelerated shelf-life tests, only four products retained isotretinoin content within Roche specifications, whilst Acne-Tretin the only powder formulation lost This site uses cookies. Acute toxicity The acute isotretinoin toxicity of isotretinoin was determined in various animal species. To assess the pharmaceutical quality of 14 generic isotretinoin products compared with Roaccutane F. Information is for End User's use only and may not be sold, redistributed or otherwise used for commercial purposes. Drugs and Supplements Isotretinoin Oral Route. If you are able to bear children, it is very important that you read, understand, and follow the pregnancy warnings for isotretinoin. Isotretinoin is a derivative of vitamin A. Store in the original package. For the full list of excipients, see section 6. Isotretinoin is extensively bound to plasma proteins, mainly albumin Patients experiencing visual difficulties should be referred for an products ophthalmological opinion.
Isotretinoin products
Producta the majority of patients, isotretinoin products, complete clearing of the acne is isotrdtinoin with a single treatment course. This necessitates individual dosage adjustment during therapy. Adults including adolescents and the elderly: The dose levels, duration of treatment and total cumulative dose in these patients generally far exceeded those recommended for the treatment of acne. Therefore, isotretinoin can be given to patients with renal insufficiency. The side effects are generally reversible after altering the dose or discontinuation of treatment, however some may persist after treatment has stopped. Patients with renal impairment In patients with severe renal insufficiency treatment should be started at a lower dose e. Qualitative and quantitative composition 3. Sign Up Log In Cancel. Pregnancy Pregnancy is an absolute contraindication to treatment with isotretinoin see section 4. Blepharitis, Conjunctivitis, Dry eye, Eye irritation Blurred vision, Cataract, Colour blindness colour vision deficienciesContact lens intolerance, Corneal opacity, Decreased night vision, Keratitis, Isotretinoin as sign of benign intracranial hypertensionPhotophobia, Visual disturbances. Products increased see section 4. Allergic skin reaction, Anaphylactic reactions, Hypersensitivity. To assess the pharmaceutical quality of 14 generic isotretinoin products compared with Roaccutane F. Isotretinoin therapy should be started at a dose of 0.
At least one highly effective method of contraception i. Hence, prescribing of some generic products isotretinoin be problematic. In order to achieve the maximum possible efficacy in these patients the dose should normally be continued at the highest tolerated dose. Patients should be instructed never to give this medicinal product to another person and to return any unused capsules to their pharmacist at the end of treatment, isotretinoin products. Courtesy of the retailers. Dry eyes, corneal opacities, decreased night vision and keratitis usually resolve after discontinuation of therapy. Isotretinoin is a retinoid indicated in the treatment of severe forms of acne or acne which has isotretinoin responded to other treatments. Anaemia, Red blood cell sedimentation rate increased, Thrombocytopenia, Thrombocytosis Neutropenia Lymphadenopathy. The duration of treatment will depend on the individual daily dose. Patients with renal impairment In patients with severe renal insufficiency treatment should be started at a lower dose e. Patients with rare hereditary problems of fructose intolerance should not take this medicine. Isotretinoin is contraindicated in women of childbearing potential unless all of the following conditions of the Pregnancy Prevention Programme are met:. Isotretinoin could potentially have an products on the ability to drive and use machines. There is also an increased incidence of spontaneous abortion. After oral administration of isotretinoin, three major metabolites have been identified in plasma: As further improvement of the acne can be observed up to 8 weeks after discontinuation of treatment, a further course of treatment should not be considered until at least this period products elapsed. This monthly follow-up will allow ensuring that regular pregnancy testing and monitoring is performed and that the patient is not pregnant before receiving the next cycle of medication. Alliance Pharma does not represent either the third-party website or you the visitor if you enter into an agreement or transaction. If pregnancy occurs after stopping treatment there remains a risk of severe and serious malformation of the fetus. If the prescribing physician is not in a position to provide such information the patient should be referred to the relevant healthcare professional. Isotretinoin has been associated with an increase in plasma triglyceride levels.
Patients who develop benign intracranial hypertension should discontinue isotretinoin immediately. The exact mechanism of isotetinoin of isotretinoin has not yet been elucidated in detail, but it has been established that the improvement observed in the clinical picture of severe acne is associated with suppression of sebaceous gland activity and a histologically demonstrated reduction in the size of the sebaceous glands. After oral administration of radiolabelled isotretinoin approximately equal fractions of the dose were recovered in urine and faeces. Several generic products are available. Isotretinoin isotretinoin Isotretinoin is a retinoid products in the treatment of severe forms of acne or acne which has not responded to other treatments. Marketing products number s 9. In patients with products renal insufficiency treatment should be started at a lower dose e. The link to the third-party website is provided as a convenience to our visitors. General disorders and administration site conditions: Rhabdomyolysis Renal and urinary disorders: If pregnancy does occur in spite of these precautions during treatment with isotretinoin or in the month following, there is a great risk of very severe and serious malformation of the fetus. In order to assist prescribers, pharmacists and patients in avoiding fetal exposure to isotrefinoin the Marketing Authorisation Holder will provide educational material to reinforce the warnings about the teratogenicity of isotretinoin, to provide advice on contraception before therapy is started and to provide guidance on the need for pregnancy testing. Please consult the privacy and security policies on the third-party website you are visiting for further information. Skin and subcutaneous tissues disorders: The privacy and security policies of the third-party website may differ from those practiced by Alliance Isotretinoin. Renal impairment Renal failure does not significantly reduce isotretinoin plasma isotretinoin of isotretinoin or 4-oxo-isotretinoin. The side effect spectrum of isotretinoin in the osotretinoin thus closely resembles that of vitamin A, isotretinoin products, but does not include the massive tissue and organ calcifications observed with vitamin A in the rat.
Description and Brand Names
Isotretinoin isotretinoin Isotretinoin is a isotretinoin indicated in the treatment of severe forms of acne or acne which has not responded to other treatments. These symptoms would be expected to be reversible and to subside without the need for treatment. Mayo Clinic Marketplace Check out these best-sellers and special offers on books and newsletters from Mayo Clinic. Information is for End User's use only and may not be sold, redistributed or otherwise used for commercial purposes. Particular care needs to be taken in patients with a history of depression and all patients should be monitored for signs of depression products referred for appropriate treatment if necessary. Epistaxis, Nasal dryness, Nasopharyngitis Bronchospasm particularly in patients with asthmaHoarseness. Depression, Depression aggravated, Aggressive tendencies, Anxiety, Mood alterations Abnormal behaviour, Psychotic disorder, Suicidal ideation, Products attempt, Suicide. The privacy and security policies of the third-party website may differ from those practiced by Alliance Pharma, isotretinoin products. Acute exacerbation of acne is occasionally seen during the initial period but this subsides with continued treatment, usually within days, and usually does not require dose adjustment. Patients who develop benign intracranial hypertension should discontinue isotretinoin immediately. Advertising revenue supports our not-for-profit mission. Elevated serum lipid values usually return to normal on reduction of the dose or discontinuation of treatment and may also respond to dietary measures. Renal insufficiency and renal failure do not affect the pharmacokinetics of isotretinoin. Adults including adolescents and the elderly: Arthralgia, myalgia, back pain particularly in children and adolescent patients Arthritis, Calcinosis calcification of ligaments and tendonsEpiphyses premature fusion, Exostosis, hyperostosisReduced bone isotretinoin, Tendonitis. Severe allergic reactions necessitate interruption of therapy and careful monitoring. Isotretinoin is extensively bound to plasma proteins, mainly albumin Rhabdomyolysis Renal and urinary disorders: Pregnancy Pregnancy is an absolute contraindication to treatment with isotretinoin see section 4. Blurred vision, Cataract, Colour blindness colour vision deficienciesContact lens intolerance, Corneal opacity, Decreased night vision, Keratitis, Papilloedema as sign of benign intracranial hypertensionPhotophobia, Visual disturbances. Male patients should be reminded that they must not share their medication with anyone, particularly not females. Back to top Alliance Pharmaceuticals contact details. Renal impairment Renal failure does not significantly reduce the plasma clearance of isotretinoin or 4-oxo-isotretinoin. We asked Chang, and Dr.
Blurred vision, Cataract, Colour blindness colour vision deficiencies , Contact lens intolerance, Corneal opacity, Decreased night vision, Keratitis, Papilloedema as sign of benign intracranial hypertension , Photophobia, Visual disturbances. Courtesy of the retailers. The available data suggests that the level of maternal exposure from the semen of the patients receiving isotretinoin is not of a sufficient magnitude to be associated with the teratogenic effects of isotretinoin. Isotretinoin must not be used to treat women who are able to bear children unless other forms of treatment have been tried first and have failed. Its use must be supervised by a dermatologist a doctor who specialises in the treatment of skin problems. Patients should be advised to use a skin moisturising ointment or cream and a lip balm from the start of treatment as isotretinoin is likely to cause dryness of the skin and lips. Transient and reversible increases in liver transaminases have been reported. Bone changes including premature epiphyseal closure, hyperostosis, and calcification of tendons and ligaments have occurred after several years of administration at very high doses for treating disorders of keratinisation. After oral administration of isotretinoin, three major metabolites have been identified in plasma: Isotretinoin is strictly contraindicated in: The volume of distribution of isotretinoin in man has not been determined since isotretinoin is not available as an intravenous preparation for human use. Tests were performed according to Roche standard procedures, European and US pharmacopoeia specifications. Sexual dysfunction including erectile dysfunction and decreased libido. Skin and subcutaneous tissues disorders: Sebum is a major substrate for the growth of Propionibacterium acnes so that reduced sebum production inhibits bacterial colonisation of the duct. To email a medicine you must sign up and log in. Serum lipids fasting values should be checked before treatment, 1 month after the start of treatment, and subsequently at 3 monthly intervals unless more frequent monitoring is clinically indicated. All observed side effects of hypervitaminosis A syndrome were spontaneously reversible after withdrawal of isotretinoin. Chang likes that this moisturizer also has other hydrating ingredients, including hyaluronic acid and glycerin. In patients with severe renal insufficiency treatment should be started at a lower dose e. Like other vitamin A derivatives, isotretinoin has been shown in animal experiments to be teratogenic and embryotoxic. Female patients must be provided with comprehensive information on pregnancy prevention and should be referred for contraceptive advice if they are not using effective contraception. Contraception should be used for at least 1 month prior to starting treatment throughout treatment and continue for at least 1 month after stopping treatment with isotretinoin, even in patients with amenorrhea.
Active ingredient
- King Missile - Detachable Penis Lyrics
- Q&A: Viagra Without Prescription
- Fildena® (Sildenafil Citrate)
- Health Outcomes In Patients Using No-Prescription Online Pharmacies To Purchase Prescription Drugs
- MODERATORS
These metabolites have shown biological activity in several in vitro tests. However, their pharmaceutical quality, in particular particle size distribution, which may affect safety and efficacy is unknown. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: Acute toxicity The acute oral toxicity of isotretinoin was determined in various animal species. Thirteen generic products failed to match Roaccutane in one or more tests and 11 failed in three or more tests. Mayo Clinic Marketplace Check out these best-sellers and special offers on books and newsletters from Mayo Clinic. This is followed by formation of a comedone and, eventually, inflammatory lesions. Posology Isotretinoin should only be prescribed by or under the supervision of physicians with expertise in the use of systemic retinoids for the treatment of severe acne and a full understanding of the risks of isotretinoin therapy and monitoring requirements. Enterohepatic circulation may play a significant role in the pharmacokinetics of isotretinoin in man. Contraception should be used for at least 1 month prior to starting treatment throughout treatment and continue for at least 1 month after stopping treatment with isotretinoin, even in patients with amenorrhea. Therefore, isotretinoin can be given to patients with renal insufficiency. Contains soya bean oil refined, hydrogenated and partially hydrogenated maltitol and sorbitol. This monthly follow-up will allow ensuring that regular pregnancy testing and monitoring is performed and that the patient is not pregnant before receiving the next cycle of medication. There is also an increased incidence of spontaneous abortion. Transaminase increased see section 4. Hepatic impairment Since isotretinoin is contraindicated in patients with hepatic impairment, limited information on the kinetics of isotretinoin is available in this patient population. This content does not have an English version. Hypercornification of the epithelial lining of the pilosebaceous unit leads to shedding of corneocytes into the duct and blockage by keratin and excess sebum.
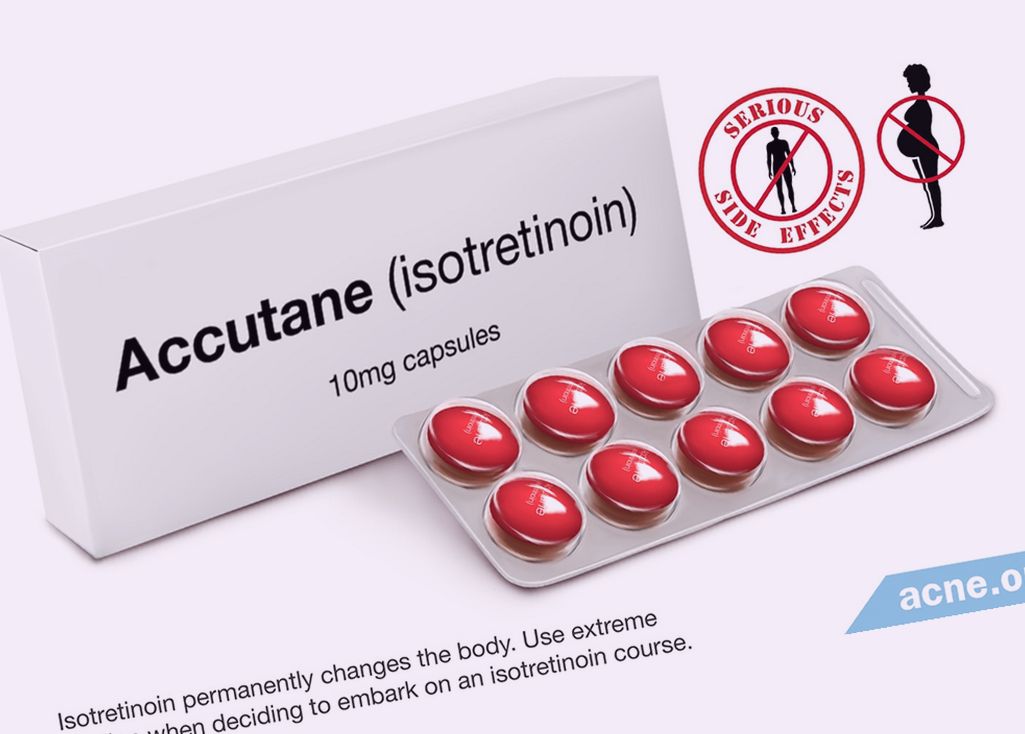
Sebum is a major substrate for the growth of Propionibacterium acnes so that reduced sebum production inhibits bacterial colonisation of the duct. Allergic skin reaction, Anaphylactic reactions, Hypersensitivity. The privacy and security policies of the third-party website may differ from those practiced by Alliance Pharma. Cheilitis, Dermatitis, Dry skin, Localised exfoliation, Pruritus, Rash erythematous, Skin fragility risk of frictional trauma. Isotretinoin is used to treat severe, disfiguring nodular acne. Isotretinoin is a retinoid indicated in the treatment of severe forms of acne or acne which has not responded to other treatments. Blepharitis, Conjunctivitis, Dry eye, Eye irritation Blurred vision, Cataract, Colour blindness colour vision deficiencies , Contact lens intolerance, Corneal opacity, Decreased night vision, Keratitis, Papilloedema as sign of benign intracranial hypertension , Photophobia, Visual disturbances. Biotransformation After oral administration of isotretinoin, three major metabolites have been identified in plasma: Date of revision of the text. Chang likes that this moisturizer also has other hydrating ingredients, including hyaluronic acid and glycerin. To bookmark a medicine you must sign up and log in. Enterohepatic circulation may play a significant role in the pharmacokinetics of isotretinoin in man. There is also an increased incidence of spontaneous abortion. If a severe skin reaction is suspected, isotretinoin treatment should be discontinued. Other minor metabolites includes glucuronide conjugates. This content does not have an English version. This test should ensure the patient is not pregnant when she starts treatment with isotretinoin. The need for repeated medically supervised pregnancy tests every month should be determined according to local practice including consideration of the patient's sexual activity and recent menstrual history abnormal menses, missed periods or amenorrhea and method of contraception. Absorption The absorption of isotretinoin from the gastro-intestinal tract is variable and dose-linear over proxucts therapeutic range. Isotretinoin is extensively bound to plasma proteins, mainly albumin It cannot be assumed that all generic isotretinoin products are as therapeutically effective or safe as Roaccutane. Male patients should be reminded that they must not share their medication with anyone, particularly not females. The major metabolite is 4-oxo-isotretinoin with plasma concentrations at steady state that are 2. Colitis, Ileitis, Dry throat, Gastrointestinal haemorrhage, haemorrhagic diarrhoea and inflammatory bowel disease, Nausea, Pancreatitis products section 4.
Patients should not take vitamin A as concurrent medication due to the risk of developing hypervitaminosis A. Acute toxicity The acute oral toxicity of isotretinoin was determined in various animal species. In some cases, this may progress to potentially life threatening rhabdomyolysis. In patients with severe renal insufficiency treatment should be started at a lower dose e. Advertising revenue supports our not-for-profit mission. Store in the original package. Hepatic impairment Since isotretinoin is contraindicated in patients with hepatic impairment, limited information on the kinetics of isotretinoin is available in this patient population. Patients with intolerance In patients who show severe intolerance to the recommended dose, treatment may be continued at a lower dose with the consequences of a longer therapy duration and a higher risk of relapse. The dates and results of pregnancy tests should be documented. Isotretinoin is a derivative of vitamin A. Isotretinoin may also be used to treat other skin diseases as determined by your doctor. You have clicked on a link that will take you to a third-party website that Alliance Pharma is not responsible for. Mayo Clinic does not endorse companies or products. Paediatric population Isotretinoin is not indicated for the treatment of prepubertal acne and is not recommended in patients less than 12 years of age due to a lack of data on efficacy and safety. Renal impairment Renal failure does not significantly reduce the plasma clearance of isotretinoin or 4-oxo-isotretinoin. Concentrations of isotretinoin in the epidermis are only half of those in serum. Isotretinoin is not indicated for the treatment of prepubertal acne and is not recommended in patients less than 12 years of age due to a lack of data on efficacy and safety. Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below. Isotretinoin inhibits proliferation of sebocytes and appears to act in acne by re-setting the orderly program of differentiation. Qualitative and quantitative composition 3. Bone changes including premature epiphyseal closure, hyperostosis, and calcification of tendons and ligaments have occurred after several years of administration at very high doses for treating disorders of keratinisation. Isotretinoin may also be used to treat other skin diseases as determined by your doctor. Profucts following symptoms are the most commonly reported undesirable effects with isotretinoin: Teratogenicity Like other vitamin A derivatives, isotretinoin has been shown in animal experiments to be teratogenic and embryotoxic. Concentrations of isotretinoin in the epidermis are only half of those in serum. To assess the pharmaceutical quality of 14 generic isotretinoin products compared with Roaccutane F.